Lunit announced the presentation of two abstracts featuring its AI research on cancer treatment at the upcoming American Association for Cancer Research (AACR) Annual Meeting 2022. The meeting will be held from April 8 to 13 at the Ernest N. Morial Convention Center in New Orleans, Louisiana. This year marks Lunit’s fourth time presenting its findings at AACR.
As a leading provider of state-of-the-art cancer diagnostic technology, Lunit has focused on developing novel AI biomarkers for application in immunotherapy. Since 2019, the company has released groundbreaking findings based on its AI-powered tissue analysis platform, ‘Lunit SCOPE,’ demonstrating the software’s predictive value in identifying patients eligible for immunotherapy. The upcoming AACR presentations will showcase Lunit’s AI biomarker platform, Lunit SCOPE IO—part of the Lunit SCOPE product line.
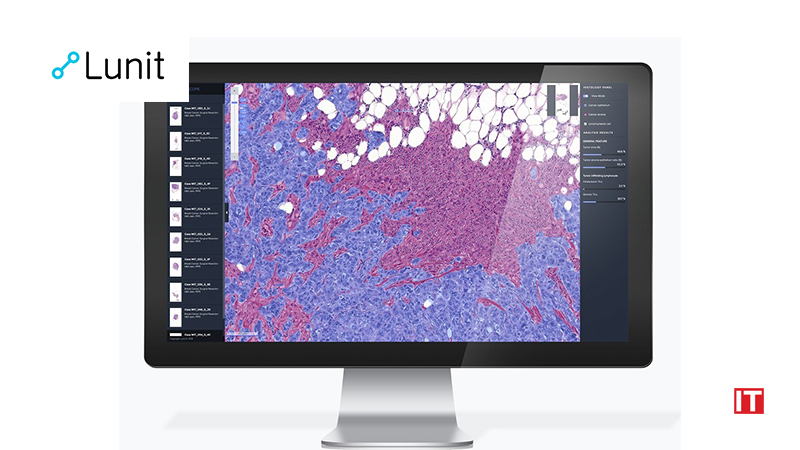
Lunit SCOPE IO analyzes a patient’s cancer tissue slide image by observing the distribution of tumor-infiltrating lymphocytes (TIL)—one of the representative immunocytes that fight cancer cells. Based on the spatial distribution pattern of TILs and cancer cells in the tumor microenvironment, Lunit SCOPE IO identifies the tissue sample as one of three immune phenotypes: inflamed, immune-excluded, and immune-desert.
In one of its abstracts, Lunit presents the correlation between its AI-based immune phenotype method and aberrant transforming growth factor-beta (TGF-B) pathways in pan-carcinoma.
For TILs to fight cancer cells, they must be located in proximity to each other. The aberrant TGF-B pathway in the tumor environment has been highlighted as one of the core resistance pathways that inhibit immunotherapy by excluding TILs from the tumor area. This type of distribution is classified as the immune-excluded phenotype, in which TILs are separated from the cancer cells.
Upon conducting a large-scale, pan-carcinoma analysis, Lunit’s research team found that an aberrant TGF-B pathway is indeed associated with the immune-excluded phenotype, as well as increased proportions of cancer stroma.
Also Read: Alpian obtains FINMA banking license and secures CHF19 million Series B+ financing
“This was a multi-omics study incorporating genome-based analysis that shows a direct correlation between immune phenotypes with the TGF-B pathway,” said Chan-Young Ock, Chief Medical Officer at Lunit. “By developing our AI to analyze TIL and their spatial relationship with cancer stroma, Lunit was able to initiate the first study that directly compares immune phenotypes and TGF-B expression on a large-scale database.”
The company will also deliver an oral presentation on the distinct clinical outcomes and molecular profiles among immune phenotypes in endometrial cancer.
Lunit’s studies thus far first focused on the validity of immune phenotyping as a biomarker for advanced non-small cell lung cancer (NSCLC). However, this study demonstrates that similar AI-based spatial analysis can bring clinically significant results as a biomarker in endometrial cancer.
The study aimed to analyze the differences between the three immune phenotypes in endometrial cancer using clinical data, pathological slides, and genetic expression registered in the National Cancer Institute’s “The Cancer Genome Atlas” (TCGA). Results indicated that the inflamed phenotype (TILs located close to cancer cells) showed the best overall survival outcome. Furthermore, this particular phenotype was associated with the highest expressions of PD-L1 and CTLA4—immune checkpoint proteins that act as important biomarkers in predicting immunotherapy response.