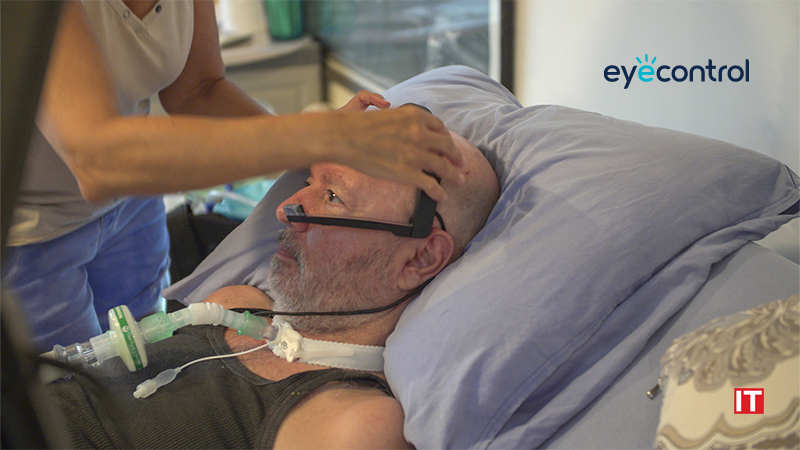
EyeControl is developing the EyeControl-Pro, an AI-based, smart, screenless and wearable, eye-tracking communication solution for Delirium reduction. The company will display its new investigational device, EyeControl-Pro, at the American Delirium Society Conference (ADS), June 12-14, Indianapolis, IN.
EyeControl-Pro is being designed to enable bi-directional remote connectivity and communication between the patient’s headset, the medical team and family members.
EyeControl CEO Mr. Or Retzkin said, “Our intention is to effectively empower the up to 80% of ICU patients experiencing Delirium to communicate with their medical support team, family and friends, center and regain control of reality. Delirium costs the US healthcare system up to $152B annually. We’ll speak about our vision and plans for Delirium management at the ADS conference.”
EyeControl will also share early data from its phase I clinical trial in Israel at Beilinson Hospital’s ICU, where preliminary results include decreases in patient errors in the Confusion Assessment Method for the ICU (CAM-ICU), a validated Delirium screening tool, as well as improvements in Lowenstein Communication Scale (LCS) scores and patient engagement with hospital staff.
Also Read: GoldenCrypto a Blockchain Technology Company, Building its own DeFi Ecosystem
EyeControl-Pro is also currently being evaluated in a multi-center, international clinical trial to investigate the safety and efficacy of the device for Delirium reduction.
Delirium is an acute mental disturbance characterized by confused thinking and disrupted attention sometimes accompanied by hallucinations; it can be brought on by anxiety, isolation and cognitive decline, which arises from lack of communication and interpersonal interactions.