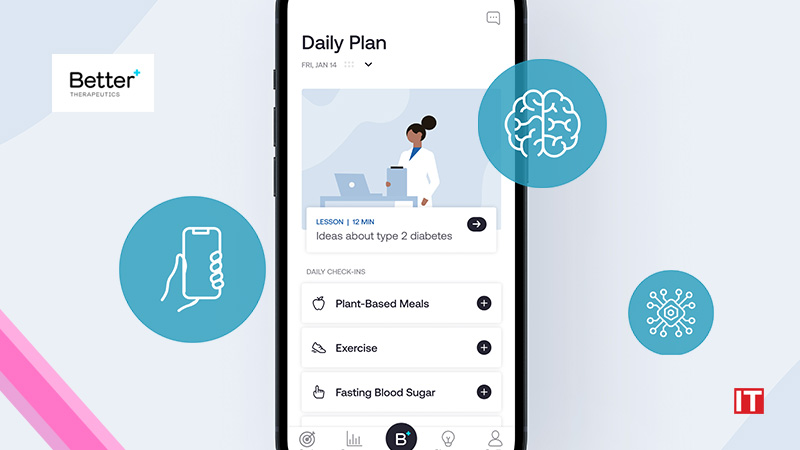
Better Therapeutics, Inc, a pioneer in developing software to treat cardiometabolic diseases, announced that the Food and Drug Administration (FDA) authorized AspyreRx™ (formerly BT-001), a prescription-only digital therapeutic (PDT) treatment indicated to provide cognitive behavioral therapy to patients 18 years or older with type 2 diabetes (T2D). AspyreRx was reviewed through the FDA’s De Novo pathway and its authorization creates a new class of diabetes digital behavioral therapeutic devices. AspyreRx is expected to launch commercially in Q4 2023.
“AspyreRx is a game-changer as we now have an evidence-based intervention to help clinicians and people living with type 2 diabetes address the underlying factors that contribute to disease progression and achieve treatment outcomes beyond glucose management alone,” said David Kerr MBChB, DM, FRCP, FRCPE, Director of Digital Health at the Diabetes Technology Society. “The cornerstone of modern diabetes care is helping to improve self-efficacy and AspyreRx now provides a prescription tool for physicians that seamlessly integrates with existing disease management programs to help patients make and sustain meaningful changes to improve their overall health.”
“This regulatory milestone signals a promising future where technology, psychology, and medicine converge to address for the first time the behavioral causes of disease for the 37 million patients living with T2D in the U.S.,” said Frank Karbe, Chief Executive Officer at Better Therapeutics. “This De Novo authorization also provides a foundation for potential future growth opportunities. Given cardiometabolic diseases share common underlying factors that contribute to their development and progression, we intend to expand our PDT platform to multiple related conditions in the future.”
AspyreRx was granted marketing authorization based on efficacy and safety data from a randomized controlled trial involving 668 participants, demonstrating clinically meaningful results, which were published in Diabetes Care.
SOURCE: Businesswire